Medical Device Consultant Online Course according to Section 83 MPDG (Mandatory Training)
Legally required online course for Medical Device Consultants – including certificate pursuant to Section 83 MPDG
Mandatory training according to Section 83 MPDG – required for individuals who:
- inform professional users about medical devices, or
- instruct users in the proper and safe use of medical devices
4-6 hours
Access anytime
Certificate
310.00 EUR
Complete the Medical Device Consultant training online
The legally required expertise in accordance with Section 83 MPDG - compact and practical

Anyone who informs professional users about medical devices or instructs them in their proper use must possess appropriate expertise in accordance with Section 83 MPDG.
This online course provides that legally required qualification in a practical, easy-to-understand format.
It prepares participants specifically for the tasks and responsibilities of a Medical Device Consultant and concludes with an official certificate.
Note: This course is intended as a basic qualification for individuals who are not yet qualified as Medical Device Consultants.
Already qualified Medical Device Consultants who wish to update their knowledge can attend our refrescher course.
Obtain the required qualification and receive a certificate
Complete the legally required continuing education at your own pace and qualify as a Medical Device Consultant.
Target group:
This course is aimed at employees of:
Medical device manufacturers
Sales organizations in the medical technology sector
Distributors and importers
who provide technical information about medical devices and, where required, instruct users in their handling.
Prerequisites: The legal requirements for working as a Medical Device Consultant are defined in Section 83 (2) MPDG.
Course type: Online self-study course
German legislation assigns Medical Device Consultants a high level of responsibility.
Their expertise plays a key role in ensuring user safety, patient protection, and the proper therapeutic use of medical devices.
Acquire the necessary qualification with Trautmann training courses.
Quick and easy registration for the online course
Select your booking method: Invoice (request form) · PayPal (instant booking) · Groups & companies (on request)
Form
-
1. complete and return the form
-
2. transfer the invoice amount
-
3. activation and receipt of access data
-
4. start the course
Book directly
-
1. create a free customer account
-
2. select the desired course
-
3. pay the participation fee with PayPal
-
4. start the course
Offer
-
Special conditions from 10 participants
-
Save money with subscription
-
Own learning platform for companies
-
Individual courses
Course structure
Content and learning objectives
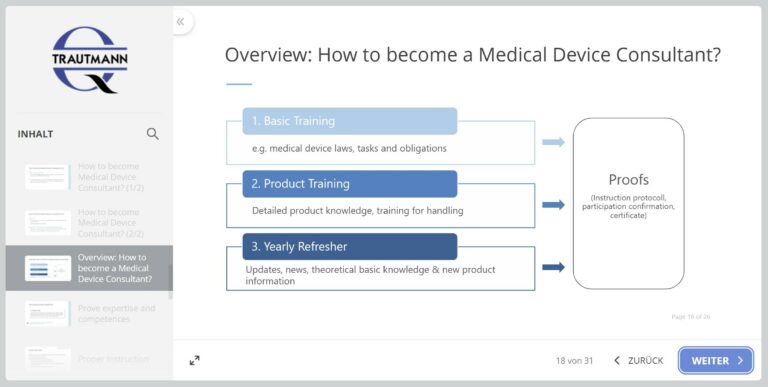
1st module: Medical Device Consultant
In the first module, you will work on the meaning, the job description and the required qualification of the Medical Device Consultant in Germany. Relevant terms (including specialist groups) are explained using examples. The focus of the learning unit is on the tasks and duties of the Medical Device Consultant. The module ends with information and recommendations for the company’s internal training concept.
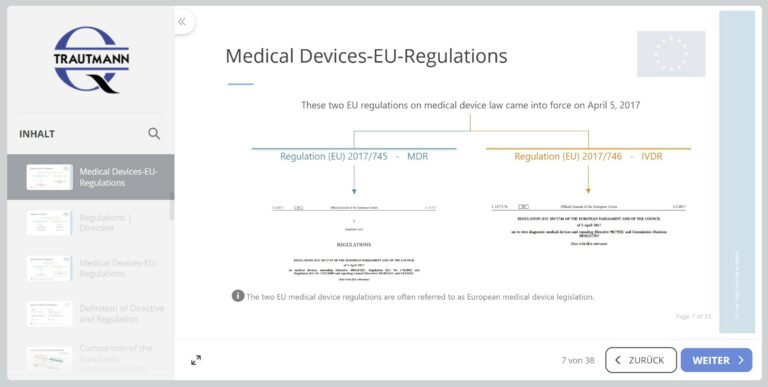
2nd module: Medical Device Law
The second module begins with an overview of European Medical Device Law. The previously applicable EU directives are mentioned and the new Medical Device Regulation (MDR) is examined in more detail. The Medical Devices EU Adaptation Act is also discussed.
3rd module: Medical devices
In this module, you will learn everything you need to know about the scope of the MDR and the distinction between medical devices and medicinal products. Another focus is on the basic safety and performance requirements. Finally, terms such as custom-made products, series products and accessories are explained.
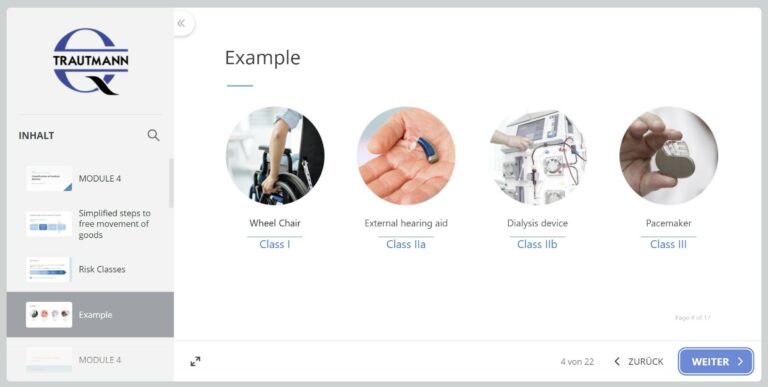
4th module: Classification
The criteria for classifying medical devices according to risk classes are discussed. Examples with explanations follow.
5th module: Conformity assessment
The fifth module provides you with an overview of conformity assessment. The contents of a declaration of conformity are also discussed.
6th module: Marking of medical devices
This module focuses on the CE mark, graphic symbols for labeling medical devices, the instructions for use, the prohibition of product-related misleading information and the unique device identifier UDI.
7th module: MPBetreibV
The seventh module covers the Medical Devices Operator Ordinance in detail. In particular, it covers instruction, the obligations of operators and users and other requirements.
8th module: Monitoring and reporting system
In this module, you will learn to distinguish between the different forms of market surveillance. You will receive an overview of the internal and external reporting system, the classification of incidents and the procedure for reporting to the competent authority.
Extract from the training documents
A varied learning approach with practical, real-world examples.



The training is also available in German.
Online Seminar: Medical Device Consultant as required by Section 83 MPDG
Flexibility during learning
There are fewer and fewer resources available for further training on the job. Time in particular is at a premium, which means that further training courses must be easy to integrate into everyday working life. Online training courses, which can be carried out individually anywhere and at any time, are therefore a good alternative to a traditional face-to-face seminar. We therefore provide you with the Medical Device Consultant training course as a fully-fledged online course, giving you absolute flexibility in terms of further training.
Learn and deepen the necessary knowledge step by step – at your own pace and with individual time management. The online course is completed with the “Medical Devices Consultant acc. to § 83 MPDG” certificate after successful completion of the test. Book the online course “Medical Devices Consultant acc. to § 83 MPDG” directly online now or submit a request for an individual offer for a face-to-face training course if you would prefer to obtain your certificate in a fixed scheduled period with a personal instructor.
Feedback from our course participants
What our customers say
Dank des übersichtlichen und aufs Wesentliche konzentrierten Aufbaus des Online-Kurses ist es mir leicht gefallen, den Kurs zu absolvieren und natürlich auch zu bestehen
Als rechtliche Grundlage beim Vertrieb eines Medizinproduktes ist dieser Kurs für mich ein Muss."
Birgit Bieg

Kinova Europe GmbH
Wir danken für die ausgezeichnete Zusammenarbeit und können das Unternehmen absolut weiterempfehlen."

HMS GmbH
Any questions about the training?
Answers regarding content, process, and participation can be found in our FAQ.
For organizational or group-related questions, we are happy to support you personally.
training courses: +49 34293 4797-27

